Focus on Research
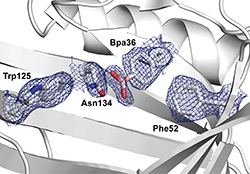
Figures. An Anticalin derived from the human lipocalin 2 scaffold was equipped with L-boronophenylalanine (Bpa) using amber suppression technology.
Top: X-ray crystallographic analysis shows that the boronate side chain protrudes from the bottom of the natural ligand cavity in a fashion that it can reversibly form diesters with cis-diol groups in various sugar compounds.
Bottom: The four surrounding loops are structurally variable and can be moulded to specifically recognize sugars (or oligosaccharides) of varying shapes.
|
Prof. Dr. Arne Skerra
B3 / Area B Synthetic Proteins
Design of next generation protein tools and therapeutics
Our group has long-standing experience in the area of protein biochemistry and protein engineering. Our goal is to understand proteins both at the structural and functional level and to design novel proteins for innovative applications as research tools, biocatalysts and as biopharmaceutical drugs for therapeutic use or in vivo tumour imaging.
Starting with the development of the first bacterial expression system for functional antibody fragments and the discovery of the Strep-tag® as a generic protein purification tool, we invented Anticalins® as a novel class of non-immunoglobulin binding proteins and PASylation technology® to tune the plasma half-life of therapeutically administered proteins and peptides. This research has led to several applications in pharma and biotech companies, including our spin-offs Pieris and XL-protein with several drug candidates in clinical trials.
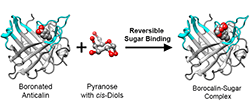
Our interest in synthetic biology aims at expanding the natural set of 20 proteinogenic amino acids in order to create proteins with unseen functionalities. We employ non-natural amino acid building blocks which serve as fluorescent probes or provide side chains with bioorthogonal chemistry (such as the keto group, which forms conjugates with hydrazines and aminooxy compounds) and with special reactivities (e.g. the boronate group, which can form cyclic diesters with sugar diol groups).
Such foreign amino acids are cotranslationally incorporated in proteins produced in E. coli using an engineered suppressor tRNA and aminoacyl-tRNA synthetase in combination with an amber stop codon that is site-specifically introduced into the coding region for the protein of interest. This strategy allows the expansion of the genetic code ond opens the route to next generation protein engineering based on biosynthetic proteins equipped with innovative side chain functionalities.
A recent example from our laboratory is the design of an Anticalin with sugar-binding function mediated by a boronate side chain that has been incorporated into its cup-shaped ligand pocket ( DOI: 10.1021/acssynbio.7b00199). Carbohydrates are camouflaged by many hydroxyl groups and, consequently are notoriously difficult to recognize and complex with high affinity by proteins. Thus, our "Borocalin" approach paves the way to a new class of sugar-binding reagents, aiming at disease-relevant specificities for applications in biomedical research and, potentially, therapy.
|
|
|
|
GRK2062 Publications
Phil. Trans. R. Soc. B 2017 372 20160380
Enhancing (crop) plant photosynthesis by introducing novel genetic diversity
Marcel Dann and Dario Leister
Abstract
Although some elements of the photosynthetic light reactions might appear to be ideal, the overall efficiency of light conversion to biomass has not been optimized during evolution. Because crop plants are depleted of genetic diversity for photosynthesis, efforts to enhance its efficiency with respect to light conversion to yield must generate new variation. In principle, three sources of natural variation are available: (i) rare diversity within extant higher plant species, (ii) photosynthetic variants from algae, and (iii) reconstruction of no longer extant types of plant photosynthesis. Here, we argue for a novel approach that outsources crop photosynthesis to a cyanobacterium that is amenable to adaptive evolution. This system offers numerous advantages, including a short generation time, virtually unlimited population sizes and high mutation rates, together with a versatile toolbox for genetic manipulation. On such a synthetic bacterial platform, 10 000 years of (crop) plant evolution can be recapitulated within weeks. Limitations of this system arise from its unicellular nature, which cannot reproduce all aspects of crop photosynthesis. But successful establishment of such a bacterial host for crop photosynthesis promises not only to enhance the performance of eukaryotic photosynthesis but will also reveal novel facets of the molecular basis of photosynthetic flexibility.
This article is part of the themed issue ‘Enhancing photosynthesis in crop plants: targets for improvement'.
Full text http: //dx.doi.org/10.1098/rstb.2016.0380
The following GRK2062 articles have also been published during the last weeks:
Selvakumar Edwardraja, Andreas Eichinger, Ina Theobald, Carina Andrea Sommer, Andreas J. Reichert, and Arne Skerra (2017) Rational Design of an Anticalin-Type Sugar-Binding Protein Using a Genetically Encoded Boronate Side Chain.
ACS Synthetic Biology
https: //doi.org/10.1021/acssynbio.7b00199
Ralph Krafczyk, Jakub Macošek, Pravin Kumar Ankush Jagtap, Daniel Gast, Swetlana Wunder, Prithiba Mitra, Amit Kumar Jha, Jürgen Rohr, Anja Hoffmann-Röder, Kirsten Jung, Janosch Hennig, Jürgen Lassak (2017) Structural basis for EarP-mediated arginine glycosylation of translation elongation factor EF-P. mBio 8:e01412-17.
https: //doi.org/10.1128/mBio.01412-17
Marion Kirchner, Kenji Schorpp, Kamyar Hadian, & Sabine Schneider (2017) An in vivo high-throughput screening for riboswitch ligands using a reverse reporter gene system. Scientific Reports, 7, 7732.
https: //doi.org/10.1038/s41598-017-07870-w
Nataliya Kitsera, Julia Allgayer, Edris Parsa, Nadine Geier, Martin Rossa, Thomas Carell, Andriy Khobta (2017) Functional impacts of 5-hydroxymethylcytosine, 5-formylcytosine, and 5-carboxycytosine at a single hemi-modified CpG dinucleotide in a gene promoter. Nucleic Acids Research
https: //doi.org/10.1093/nar/gkx718 |
|
|
|
New Members
PhD-students
 |
Stefan Marchner, M.Sc., started his PhD study in August 2016. Supervised by Anja Hoffmann-Röder he is focusing on “Proteomic investigation and identification of novel O-GlcNAc modified proteins and their role in epigenetics”. |
|
|
|
|
|
Farewells
Marion Kirchner defended her PhD thesis in September 2017 and graduated with distinction. Marion was working as PhD-student in the Schneider lab. We wish her all the best for her future career and of course we stay in touch! |
|
|
|
Events
 |
|
For this year's retreat, our GRK 2062 teamed up with two other European graduate schools with very similar scientific aims: The Research Center for Functional Molecular Systems from the Netherlands and the NCCR Molecular Systems Engineering from Switzerland. The schools jointly organized a pre-conference taking place at the University of Basel before the first International Conference on Molecular Systems Engineering (ICMSE 2017). During this pre-conference, the PhD students and postdocs were joined by Benjamin Davis, Vincent Noireaux and Roy Bar-Ziv, three internationally renowned researchers covering various aspects of synthetic biology. In addition to their keynote lectures, inspiring talks were given by selected postdoctoral researchers and PhD candidates of the three programs. During the poster session, posters by GRK 2062 members Martin Rossa and Chiara Gandini stood out and were selected for poster prizes. Beyond science itself, there were sessions about scientific publishing, the philosophy of life sciences, working in Start-Ups and a most entertaining science slam performed by members of the graduate programs, followed by a pizza party. In summary, the pre-conference offered great opportunities to present our science in a relaxed environment, and was especially instrumental in getting to know fellow PhD students from the other two graduate schools. The already sleep-deprived junior researchers then continued to attend the main conference, which featured some of the most esteemed researchers of our field. Another highlight was the conference dinner at the Roche tower that followed a keynote lecture by Nobel laureate Stefan Hell. And between all of this, the GRK members even found some time to get to know the charming city of Basel.
|
|
Group Photo:
ICMSE Pre-Conference
Uni Basel, Switzerland
26-27 August 2017
|
|
Report: RISE Program
Caroline Blassick (awardee): This summer, I had the opportunity to work at LMU through an internship funded by the DAAD. This DAAD program, known as RISE, matches American, Canadian, and British university students with PhD candidates in labs across Germany, where they collaborate on a project over the course of a summer. I was matched with Mona Dotzler, a PhD student in the Papenfort Lab at the LMU Biocenter.
Our project focused on uncovering the role of a small regulatory RNA in the bacterium Vibrio cholerae. This RNA is known as Vcr082 and is highly expressed at low cell density. I gained several insights into the behavior of Vcr082, such as its ability to inhibit the expression of cholera toxin. This regulation occurs at the RNA level rather than through the short peptide that Vcr082 encodes for.
In addition, I gained an understanding of what the scientific community in Germany is like. There were surprisingly few differences between the Papenfort lab and my lab in my home country — and wherever differences did arise, they taught me lessons about how to improve as a researcher. That's why this collaboration was so valuable; I believe we all can learn much from one another!
Mona Dotzler: It was a pleasure to have Caroline around for the summer and also the project moved a big step forward. For me as a supervisor, it was a very interesting experience to work with a student from a different educational background. If you consider taking part in the RISE program, feel free to contact me with any questions. |
|
RISE trainee,
participating in the Papenfort lab staff outing, summer 2017
|
|
Upcoming Transferable Skills Courses
Effective communication: how to get to the point, Sabine Walter
17th and 18th of November, 2017.
In this course, you will get to know an easy communication structure which will help you to communicate in a convincing way. You will become conscious of body language and master your emotions even in critical situations. Furthermore, you will learn how to give criticism constructively without offending or creating conflict. You work with two Executive Coaches supported by Camcorder. After every recorded video, you will get individual personal feedback from one of the coaches. More details
Scientific Writing, Science Craft
This course will run on the 22nd and 23rd of February, 2018. Course description
Effective Visual Communication for Scientists, Seyens
Attention: course postponed to spring 2018
If created properly, graphics are the most effective way to explain complex ideas in the shortest amount of time, attract audience and raise credibility. Nevertheless, researchers aren't trained in visual communication in the traditional PhD curricula and are supposed to acquire these skills by themselves. This workshop uses a hands-on approach to help researchers visually present their own research through various means of scientific communication.
|
|
|
|
Journal Club
Methods Mol Biol. 2017; 1651: 263-273
Article
Design of Synthetic Promoters for Gene Circuits in Mammalian Cells
Saxena P, Bojar D, Fussenegger M
Synthetic biology, the synthesis of engineering and biology, has rapidly matured and has dramatically increased the complexity of artificial gene circuits in recent years. The deployment of intricate synthetic gene circuits in mammalian cells requires the establishment of very precise and orthogonal control of transgene expression. In this chapter, we describe methods of modulating the expression of transgenes at the transcriptional level. Using cAMP-response element-binding protein (CREB)-dependent promoters as examples, a tool for the precise tuning of gene expression by using different core promoters and by varying the binding affinity of transcription factor operator sites is described.
Full text: http: //dx.doi.org/10.1007/978-1-4939-7223-4_19 |
|
|
|
Miscellaneous
Responsible Research - Toolbox
On July 20, about 250 participants took part in a great event engaging in good scientific conduct especially focusing on reproducible research.
As part of the take home message the organizers and speakers put together additional information about Good Scientific Practice in the form of a toolbox. |
|
|
|
Publisher
GRK 2062 Molecular Principles of Synthetic Biology
Ludwig-Maximilians-Universität München
LMU Biocenter
Großhaderner Str. 2-4
82152 Martinsried
Germany
Editor
Dr. Beate Hafner
Contact
E-Mail: grk2062 @bio.lmu.de
Phone: + 49 89 2180-74714
Web: http: //www.grk2062.lmu.de |
|
|