Focus on Research
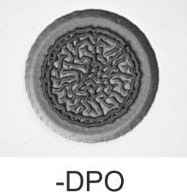

Figure 1 DPO inhibits biofilm formation in V. cholerae.
V. cholerae cells were grown in liquid medium to exponential phase prior to being spotted onto an LB agar plate lacking or containing 1 mM DPO.
Above: Biofilm formation without DPO.
Below: Biofilm formation with DPO
|
Prof. Dr. Kai Papenfort
A5 / Area A Synthetic Cells and Switches
A novel communication molecule controlling biofilm formation and virulence gene expression in V. cholerae
Quorum sensing (QS) is a cell–cell communication process that allows bacteria to modify their behavior in response to changes in the cell number and species composition of the vicinal community. Many, if not all, bacteria use QS to orchestrate the gene expression programs underlying collective behaviors. QS depends on the production, detection, and response to extracellular signaling molecules called autoinducers (AIs). In Vibrio cholerae, the causative agent of cholera disease, QS is intimately linked to biofilm formation and virulence gene expression. Two well-studied AIs, AI-2 (4,5-dihydroxy-2,3-penanedionine) and CAI-1 ((S)-3-hyroxytridecan-4-one), are known to control QS of V. cholerae. Both AIs channel information through one signal transduction pathway controlling the production of four homologous non-coding RNA, which are the key regulators of QS in V. cholerae.
In our recent work, we have identified a novel QS pathway in V. cholerae. The AI of the system is DPO (3,5-dimethylpyrazin-2-ol), a new molecule to biology and the first pyrazine involved in QS. In V. cholerae (and other Vibrios), DPO is sensed by the cytoplasmic LuxR-type transcriptional regulator, VqmA, which in turn activates the transcription of the VqmR small regulatory RNA (sRNA). VqmR regulates multiple target genes, including the mRNAs encoding the RtxA toxin and the major biofilm regulator, VpsT. Indeed, we discovered that synthetic DPO efficiently represses biofilm formation as well as virulence gene expression in V. cholerae.
|
|
|
|
GRK2062 Publications
Open Biol. 2018 8
Bacterial transmembrane signalling systems and their engineering for biosensing
Kirsten Jung, Florian Fabiani, Elisabeth Hoyer and Jürgen Lassak
Abstract
Every living cell possesses numerous transmembrane signalling systems that receive chemical and physical stimuli from the environment and transduce this information into an intracellular signal that triggers some form of cellular response. As unicellular organisms, bacteria require these systems for survival in rapidly changing environments. The receptors themselves act as ‘sensory organs’, while subsequent signalling circuits can be regarded as forming a ‘neural network’ that is involved in decision making, adaptation and ultimately in ensuring survival. Bacteria serve as useful biosensors in industry and clinical diagnostics, in addition to producing drugs for therapeutic purposes. Therefore, there is a great demand for engineered bacterial strains that contain transmembrane signalling systems with high molecular specificity, sensitivity and dose dependency. In this review, we address the complexity of transmembrane signalling systems and discuss principles to rewire receptors and their signalling outputs.
Full text https://doi.org/10.1098/rsob.180023
|
|
|
|
New Members
PhD-students

Franziska Koller, M.Sc. Biology, is supervised by Jürgen Lassak since February 2018. Working title of her PhD thesis: “Novel protein deoxyhexose modifications in bacteria: evolution and function of EF-P rhamnosylation”. |

Tianhe Wang, M.Sc. Biology, started his PhD study in November 2017. Supervised by Fritz Simmel he is working on dynamics of synthetic gene circuits in vitro and in vivo.
|
PostDocs

Dr. Alejandro Torrado joined the Leister lab in May 2017. His research is focused on the introduction of plant complexes into the cyanobacteria Synechocystis sp. in order to study the experimental evolution of these complexes under stress conditions. |
|
|
|
Events
 |
|
The third Conference on Synthetic Biology will take place from July 23 - 25, 2018 in Landshut. Registration is still open till middle of June, abstract submission has already expired.
The conference program consists of a great mixture of invited speakers talks, selected short talks of participants as well as poster sessions. Social events like the sightseeing tour in Landshut, a Bavarian buffet resp. a barbecue in the evening will provide a pleasant and relaxed atmosphere for fruitful scientific discussions between all participants and the speakers.
Upcoming Transferable Skills Courses
Grant Proposal Writing by Brian Cusack and Babette Regierer
The course will run on the 18th and 19th of June, 2018. For a full description please click here. Participation will be awarded with 1.0 ECTS.
Registration: Please write an e-mail to GRK2062
Selfmarketing for women by Sabine Walter and Greta Wonneberger
For a full description please click here. Participation will be awarded with 1.0 ECTS.
Dates:
27th and 28th of June, 2018 (fully booked)
8th and 9th of November, 2018
Registration: Please write an e-mail to GRK2062
Effective Visual Communication for Scientists, by Seyens
September 20-21, 2018
If created properly, graphics are the most effective way to explain complex ideas in the shortest amount of time, attract audience and raise credibility. Nevertheless, researchers aren't trained in visual communication in the traditional PhD curricula and are supposed to acquire these skills by themselves. This workshop uses a hands-on approach to help researchers visually present their own research through various means of scientific communication.
Participation will be awarded with 1.0 ECTS.
Registration: Please write an e-mail to GRK2062 |
|
|
|
Journal Club
Science 13 April 2018: Vol. 360 no. 6385 p. 150-151
Perspectives
Improved memory devices for synthetic cells
Joanne M. L. Ho and Matthew R. Bennett
Synthetic biologists have long sought to make cells more like computers. This is not because they think cells will be more efficient than silicon—current microelectronics make excellent computers and are less messy than cell cultures—but instead because synthetic cells can interface with biology to perform biochemical tasks. Synthetic cells might one day be capable of attacking tumors or releasing site-specific drugs inside the human body. But to carry out these tasks, synthetic biologists must be able to program cells much in the same way we program computers—by providing them with decision-making capabilities based on inputs. Indeed, prototypes of many of the genetic parts necessary for turning cells into biocomputers have been constructed, including transcriptional logic gates (1), timers (2, 3), counters (4), memory devices (5, 6), tunable sensors (7, 8), and even in vitro DNA systems that can perform complex calculations (9). On page 169 of this issue, Tang and Liu (10) expand the capabilities of cellular computers by engineering a new memory device that records events directly onto DNA.
Full text: http: //dx.doi.org/10.1126/science.aat3236
Science 13 April 2018: Vol. 360 no. 6385 p. 150-151
Article
Rewritable multi-event analog recording in bacterial and mammalian cells
Weixin Tang and David R. Liu
We present two CRISPR-mediated analog multi-event recording apparatus (CAMERA) systems that use base editors and Cas9 nucleases to record cellular events in bacteria and mammalian cells. The devices record signal amplitude or duration as changes in the ratio of mutually exclusive DNA sequences (CAMERA 1) or as single-base modifications (CAMERA 2). We achieved recording of multiple stimuli in bacteria or mammalian cells, including exposure to antibiotics, nutrients, viruses, light, and changes in Wnt signaling. When recording to multicopy plasmids, reliable readout requires as few as 10 to 100 cells. The order of stimuli can be recorded through an overlapping guide RNA design, and memories can be erased and re-recorded over multiple cycles. CAMERA systems serve as “cell data recorders” that write a history of endogenous or exogenous signaling events into permanent DNA sequence modifications in living cells.
Full text: http: //dx.doi.org/10.1126/science.aat3236 |
|
|
|